Flixotide Product Page Flovent Product Page
If you or someone you know has asthma, there is a good chance they may be prescribed an inhaled medication to help prevent asthma attacks. These medications are used daily to help reduce the inflammation in your lungs and help prevent asthma attacks, along with the shortness of breath, wheezing and discomfort associated with it.
What’s in them?
The main ingredients in most inhaled medications are corticosteroids. In our body these naturally produced hormones are made by the adrenal glands and help control inflammation.
Fluticasone, a corticosteroid, is a synthetic (i.e. man-made) form of this hormone and works to help reduce the inflammation in your lungs by inhibiting the release of allergic and other response chemicals in the body such as histamines, cytokines and leukotrienes. As a result your lungs and air passages remain open so breathing is easier.
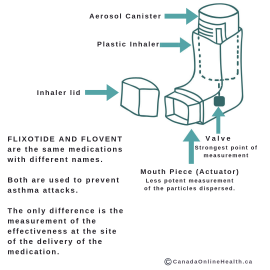
Fluticasone is found in both Flixotide™ AND Flovent™.
How is it that the strengths are labelled differently and you are telling me they are the same?
Simply put – the difference is where the medication delivered to the patient is measured. While the medications are the same, the difference in the labelled strengths is due to the measurement at a different point in the device.
When the inhaler delivers the medication, 50 mcg is delivered from the valve (upstream), but only 44 mcg from the actuator (mouth piece). The actual amount delivered to the patient lungs is variable and depends on good inhalation technique.
The regulatory authorities in the US chose the amount of medication delivered from the actuator as the labelled inhaler strength (i.e. 44 mcg, 110 mcg and 220 mcg). The New Zealand, Canada and UK labelled strength reflect the amount dispensed from the valve of the same device.
One Product with Two Different Names – It Just Depends on Where You Buy It
It may be hard to believe that one product can have two different names depending on which country you live in, but it’s true. Flixotide™ and Flovent™ inhalers are the same medications, manufactured by GlaxoSmithKline – and contain the same active ingredient, which is fluticasone propionate. While you may wonder if they are two different medications or generic equivalents of each other, they are not.
Flixotide™ inhaler – Available in the UK and New Zealand
Flixotide™ or Flixotide Evohaler™ comes in small pressurized canister and is inserted into a plastic inhaler (actuator) which provides measured doses of fluticasone propionate. Flixotide™ is available in New Zealand and the UK and is called Flovent™ in Canada. The inhaler is available in 3 labelled strengths: 50 mcg, 125 mcg and 250 mcg. This is the equivalent to the labelled strengths 44 mcg, 110 mcg and 220 mcg of Flovent™ in United States, respectively.
Fluticasone aerosol and powder brand names explained:
The table below outlines the brand name fluticasone inhalers in disc and aerosol formats.
| Fluticasone Inhaler Options | Disc Style Inhaler | Aerosol Inhaler | Country Available |
| Flovent Diskus™ | Yes | Canada and USA | |
| Flovent HFA™ | Yes | Canada and USA | |
| Flixotide Accuhaler™ | Yes | UK and New Zealand | |
| Flixotide Evohaler™ | Yes | United Kingdom | |
| Flixotide Inhaler™ | Yes | New Zealand |
Flovent HFA™ is the liquid form of fluticasone, which comes in an inhaler. The inhaler creates a spray mist that is inhaled through the mouth and into the lungs an dis also appropriate for pets. Flovent Diskus™ contains the powdered form of fluticasone which must be inhaled using the power of your breath. The disc style inhaler opens and loads the measured amount of fluticasone for each dose. The disc style inhalers are not appropriate for pets.
If you have questions about your prescription or non-prescription medication, please contact the team at Canada Online Health by calling toll free 1-800-399-DRUG (3784) or visit their website at https://www.canadaonlinehealth.ca. One of the friendly and discreet pharmacy representatives will be happy to answer your questions.
This article contains medical information provided to help you better understand this particular medical condition or process, and may contain information about medication often used as part of a treatment plan prescribed by a doctor. It is not intended to be used as either a diagnosis or recommendation for treatment of your particular medical situation. If you are unwell, concerned about your physical or mental state, or are experiencing symptoms you should speak with your doctor or primary health care provider. If you are in medical distress please contact emergency services (such as 911).